Even an organization with stellar leadership and a solid core of employees experiences hiccups from time to time. Despite having assembled all the ingredients for a great organizational culture or a superior product, a company or department is not likely to have everything perfected at the outset.
However, in the pharma and healthcare industries, a lower tolerance for error exists because mistakes have a greater potential to damage human life. Therefore, the Institute of Medicine also recommends healthcare organizations pay critical attention to their quality management systems with an eye for intervention and improvement.
There is the famous maxim that says to err is human, but to persist is diabolical. Therefore, all organizations, especially healthcare organizations, should run continuous improvement campaigns to stop mistakes from persisting. Keep scrolling through the blog article to understand the continuous improvement concept and how you can implement it in your organization.
What Exactly is the Continuous Improvement Concept?
Continuous improvement, sometimes called continual improvement, is the ongoing improvement of products, services, or processes through incremental or breakthrough improvements. These efforts can seek "incremental" improvement over time or "breakthrough" improvement all at once.
Although the terms Continuous improvement and Continual improvement are used interchangeably, continual improvement refers to improvement actions taken at regular, frequent, repeated intervals. On the contrary, continuous improvement may not necessarily occur in pre-planned intervals. Therefore, an organization that adopts a policy of reviewing products, processes, or services at regular intervals to find improvement opportunities is seeking Continual improvement. Whereas an organization that adopts a policy to improve products, processes, or services as and when complaints or adverse events happen is seeking continuous improvement. However, in either case, the improvement is implemented without cessation of the concerned product, process, or services. Therefore, we will refer to both terms as Continuous improvement in the present article for simplicity.
Best businesses utilize the Continuous improvement concept in quality management science to keep their products and employment teams at their peak performance levels with respect to quality, cooperation, performance, and engagement. The pharma and healthcare industries also derive enormous benefits from adopting a continuous improvement model that seeks a gradual increase in quality over time and often even achieves breakthrough levels of improvement within shorter periods.
The business world utilizes several quality management models that drive continuous improvement cycles through well-defined and time-bound goals. Dr. William Deming's Plan-Do-Check-Act (PDCA cycle) is one such continuous improvement model used extensively by quality managers across the globe. The popularity of the PDCA model is high owing to its simplicity and demonstrated history of achieving positive results.
In this article, we will discuss the PDCA model, an extended version of the PDCA model known as the OPDCA model, and a few continuous improvement case studies in the life science industry.
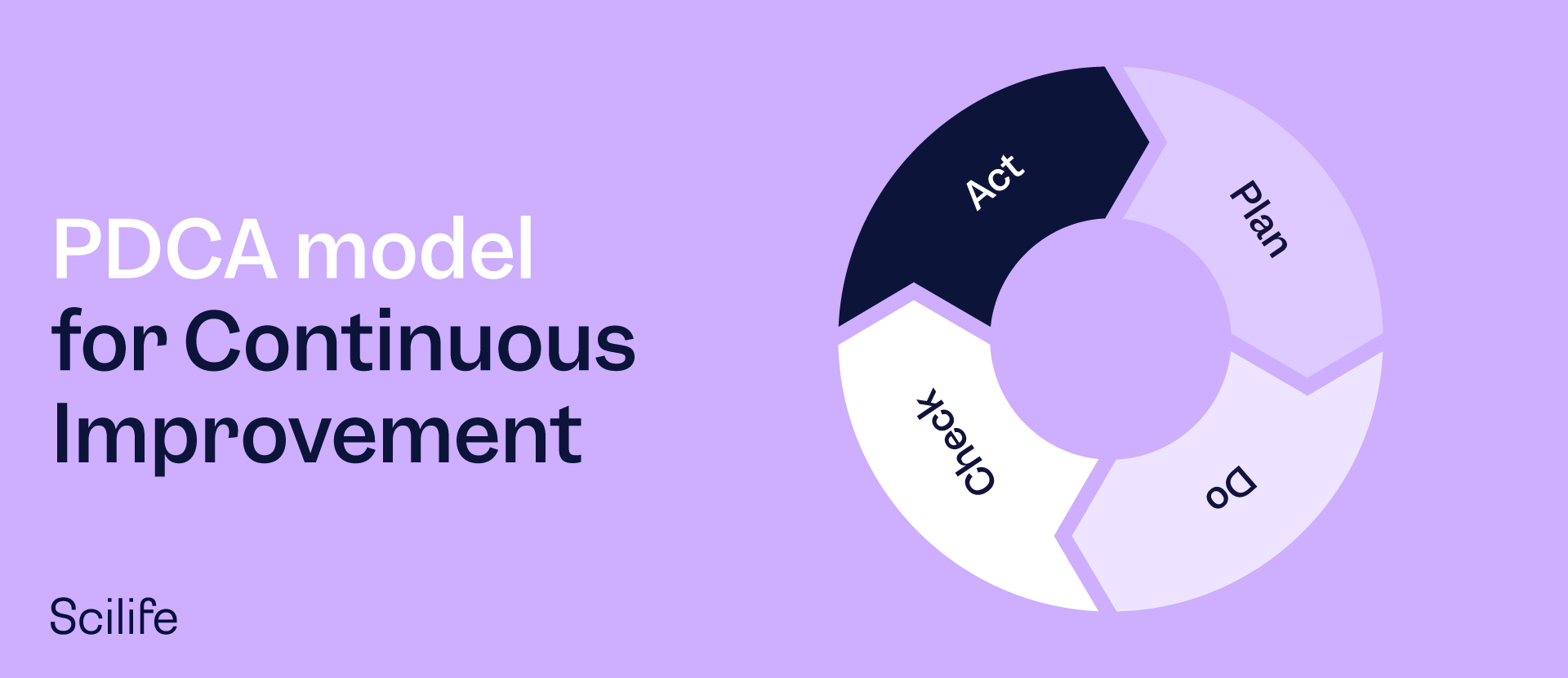
The PDCA model for Continuous Improvement
PDCA—the Plan-Do-Check-Act Model—involves identifying a problem in the organizational setting and implementing a continuous improvement strategy that involves the four steps of Plan-Do-Check-Act in a continuous cycle as follows:
1. Plan
This involves devising an initial plan to improve the product, process, or service. It may include collecting all data about the issue, followed by a strategy for testing hypotheses to determine the root causes.
2. Do
This means implementing a plan that has been proposed as a possible solution. This implementation is a test of the proposed solution, and during this stage, the team measures the results to gauge the effectiveness of the intervention.
3. Check
This is about verifying the data before-and-after implementation of the Plan to compare the performance of the improved process Vs. the old process. At this stage, the group or manager decides whether the initial assessment and hypothesis about how to improve were in the right direction.
4. Act
This refers to planning and documenting future actions based on success or failure at the check stage. So, if the tested plan works, it gets implemented throughout the organization, and if it doesn't work, it gets adjusted, and the continuous improvement cycle is re-entered.
Expanding PDCA to OPDCA for Continous Improvement
OPDCA is a more scientific form of PDCA as it adds an observation stage prior to the other phases. This updated OPDCA model is useful not only for advancing quality but also for advancing intelligence, sustainability, and performance goals. Managers who use the OPDCA model spend time learning and understanding the environment by observing how things are meant to occur and the deviations that might cause hiccups within the system.
Since the health sector relies on experimental results, the extension of the system to include an observation step is particularly pertinent to the industry. The observation must be performed first to collect the data for devising a plan (or hypothesis). After successfully devising a plan, the quality cycle continues through the Do-Check-Act phases, as described above.
Recent research by Mazzocato et al. has shown that the healthcare and pharmaceutical sectors need to strengthen their regular quality assurance systems with continuous improvement methods to enable employees and managers to address complex issues, such as the improvisation of clinical care processes. This focuses on processes rather than the individual to demonstrate the importance of examining systems functioning for engineering operational solutions.
Non-conformity as Continuous Improvement Driver
One surprising but critical factor that supports continuous improvement is the existence of deviations, complaints, and instances of non-conformity within the organization's processes. The term non-conformity is used when performing quality audits to determine whether a system, product, or process fails to meet all the standards or specifications outlined in its charter or objectives. In the health industry, this may refer to instances within the system that may put a patient's health at risk due to compromised safety and efficacy of the medication or medical device, privacy breaches, or physical dangers imposed by violating GxP compliance inside the facility.
Viewing Problems as Continuous Improvement Opportunities
Whenever clients, patients, or employees file complaints or such non-conformity is otherwise shown to exist, rather than consider it a failure of the system, managers and quality control specialists view it as an opportunity to improve the system so that it attains its best-in-class performance capabilities. Sometimes, continuous improvement may not be triggered by non-compliance but due to unmet patient needs owing to longer manufacturing times, supply chain gaps, and other miscellaneous issues.
While preparing a performance report to log such problems, the document should be composed with an eye for potential improvement opportunities. There are countless examples of how big life science players have turned their problems into opportunities by embracing the continuous improvement work culture. Pfizer's continuous improvement achievement is an excellent example of how organizations can turn problems into opportunities. Let's explore the example to draw some inspiration.
Pfizer's Continuous Improvement Achievement
Goal:
Reduce the end-to-end lead time to deliver Lipitor by 75% in two phases. In the first phase, the end-to-end lead time was aimed to be reduced by 30% within a year, followed by the next 45% in the second year.
Team Involved:
Manufacturing, Logistics, and Transportation Support Colleagues.
Approach:
The team began their work in April 2007. Pfizer utilized the Value Stream Mapping concept in Lean Philosophy to reduce the end-to-end lead time. Their initial step was mapping the value stream of those processes by which Lipitor evolves from raw material to packaged tablet; the team was able to identify the specific points at which bottlenecks, redundancies, downtime, and other non-value–added problems impeded process effectiveness and undermined process capability. Bottlenecks were found to be associated with poor process capability or unreliable equipment. In this manner, Pfizer's Lipitor team used the problem of unmet patient needs as an opportunity to make more efficient processes.
Result:
The team's first goal was to reduce lead time by 30% by year's end; they achieved this milestone by December 2007. Next, the team worked in collaboration with colleagues across several Pfizer sites to achieve another milestone by the end of 2008: trimming end-to-end Lipitor lead-time by 60%.
Conclusion
In the life science industry, continuous improvement cycles provide a significant benefit to the patients, as these improvements are directly associated with safety, efficacy, quality, and unmet patient need. Developing a problem-solving attitude while dealing with challenges in product development or services creates opportunities for increased efficiency.
The organization and its managers dedicated to processual and incremental improvement get the best out of any situation, including instances of non-conformity. For such an organization and its managers, instances of deviation are opportunities in order to refine the process and make it even safer, more efficient, and more satisfying to the company's clients, employees, stakeholders, and the public at large.
Discover how a Smart QMS can help you easily implement the Continuous Improvement Cycle